this looks like most of the ones we could actually be assessed on (theres also a fermentation prac but it would take to long and i doubt very much sir would do it)
Molar heat of combustion:
Galvanic Cells:
Potential difference of metals in an electrode:
Natural Indicators:
Decarbonation of a soft drink:
Titrations:
thanks so much jake your a life saver
Let's do thisMolar heat of combustion:Here, we are trying to extract the heat released by combusting one mole of a substance. For arguments sake, let's assume your prac is Ethanol, however it could foreseeably be any other alcohol.
We look at the formula, which is

This will tell us how much energy has been released, depending on the increased temperature of the water.
We set up the below prac
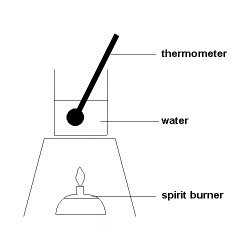
First, we weight the mass of the substance (eg. Ethanol). Then, we light it, and let it heat up the water. Finally, we extinguish the Ethanol, measuring A) the change in mass of the Ethanol and b) the change in temperature of the water.
Let's say the water heated up by 20 degrees, and one gram of Ethanol was combusted. First, we find the energy that must have been 'used' to heat up the water by this amount, using the formula above
)
Where m is the mass of the water, and C is the specific heat capacity. This will give us some value of H, which was released when one gram of Ethanol was burned. Then, we convert this kJ/g measurement into kJ/mol (my multiplying by the molar mass), and we have a total value!
Some notes on the 'reliability' etc part of this experiment. Firstly, we would perform it multiple times, and average the result. Second, validity is quite limited in this experiment for two reasons: 1) A lot of heat energy is lost to the environment and 2) incomplete combustion can occur (if there is a black smudge underneath the tripod. These are all things you would talk about in the written portion of the prac.
One down. Like 100 to go.
Galvanic Cells:For a Galvanic Cell, you need to be really confident re how to draw one out/set one up/label it. The below is a bit of a blurry example, but you get the idea.

So, set it up by placing the liquids in two beakers, and the appropriate electrode in each beaker, connected to a voltmeter in series. Also, make sure to connect the two half cells with a salt bridge, to allow ions to travel between (essentially completing the circuit!).
For an experiment like this, there isn't much to it. You might be asked to change the concentration of the solution, and measure the affect of this on the voltage etc. You don't need to know anything about any possible variances in advance; you'll have to actually do it on the day.
Be comfortable with half-cell notation, redox equations, etc.
I've never heard of a practical task that was a Galvanic cell, so I think this is pretty unlikely
 Potential difference of metals in an electrode:
Potential difference of metals in an electrode:This is the same as a Galvanic cell. Potentially (lol) you'll need to switch metals, compare the output voltage etc. This can all be predicted using the table of standard potentials on your formula sheet; you find the was oxidising, and switch the signs of the potential on the sheet. Then, you add it to the one reducing, and presto! You've got a voltage out. So, the highest voltage would be a cell between a substance very high on the list, and a substance very low on the list. Be comfortable with this table, with balancing equations, which creating net ionic equations and half equations. Happy to give some examples if you think that will help.
Natural Indicators:Also a pretty straight forward practical task. Usually, you'll use something like red cabbage. Here's how I did the prac.
Cut up the cabbage into smallish pieces, and place it in a beaker full of water. Smoosh it around, so it releases its delicious delicious juices. Then, boil it for about 5 minutes, also to ensure that you get it's sick ass ability to detect changes in pH. Drain the remaining liquid, so you retain only the solution, and throw away the soggy Cabbage.
Now, you've got your indicator! We need to test how useful it is, and what range it will be able to recognise. So, prepare a range of test tubes (ideally, multiple at each pH scale for reliability) such that each test tube has a different pH. I would try to get a test tube with pH 1, a test tube with pH 2.... a test tube with pH 12 and a test tube with pH 13. If not possible, just ensure you have the full range. Drip a few drops of the indicator into each test tube, and record any colour change. It will be obvious what 'sort' of indicator yours is once you've done this! ie. if it's red for all substances up until a pH of 9, and then blue for pHs higher than 10, it would be useful in determining if something is very basic.
Ooft. Getting there.
Decarbonation of a soft drink:This one is pretty chilled. However, there is a bit of nuance to it. First, you'll get a can of soft drink. Weigh it. Then, place it on a scale, and open the can. MAKE SURE NOT TO RIP THE TAGGY BIT OFF, OR YOU'LL BE CHANGING THE WEIGHT! Let the soft drink sit, and record changes in mass over whatever time period you have (if it's an assessment, probably not long). Plot this change in weight against time, and you'll have a nice curvey/straight lineish graph.
What's happening is that Carbon dioxide is being released. Based on the change in mass, you can calculate exactly how much Carbon dioxide has been released, based on its molar mass. I assume you can do this calculation.
Now, if you want to be really smart, consider the main issue with validity. So, you're watching Carbon dioxide escape, but what else?
Water is evaporating. So, if you want to be clever, get a different can, empty it, and fill it with water. Simultaneously measure any change in mass in this can, which would be entirely due to water loss. Approximate that the evaporation in the second can is the same as the first, and 'add' the additional weight to your data. eg. if you notice that the water can loses 3g, but the soft drink can loses 5g, you can only actually attribute 2g to Carbon dioxide escaping.
Remember that this is all done in terms of Le Chatelier's principle, although the experiment itself doesn't seem to involve it. Just be comfortable with the principle and it's applications in order to answer any theoretical questions.
Titrations:AAAAAAAND now we get to the tough one. Let me see if I have any helpful images...
I DO INDEED!

Hell yeah. This genuinely has everything you need. The LHS is about creating a standard, the RHS is about performing the titration.
I think it's actually fairly comprehensive; let me know if I can elaborate on this at all.
Phew.

