Thank you guys 
You said that the reason why detergent dissolves in water is because it has a hydrophilic head and hydrophobic tails. Then why is it that our phospholipids are not the same as they also have hydrophilic heads and hydrophobic tails? Could it be because they don't form micelles? If so, then why do detergents form miscelles in the first place?
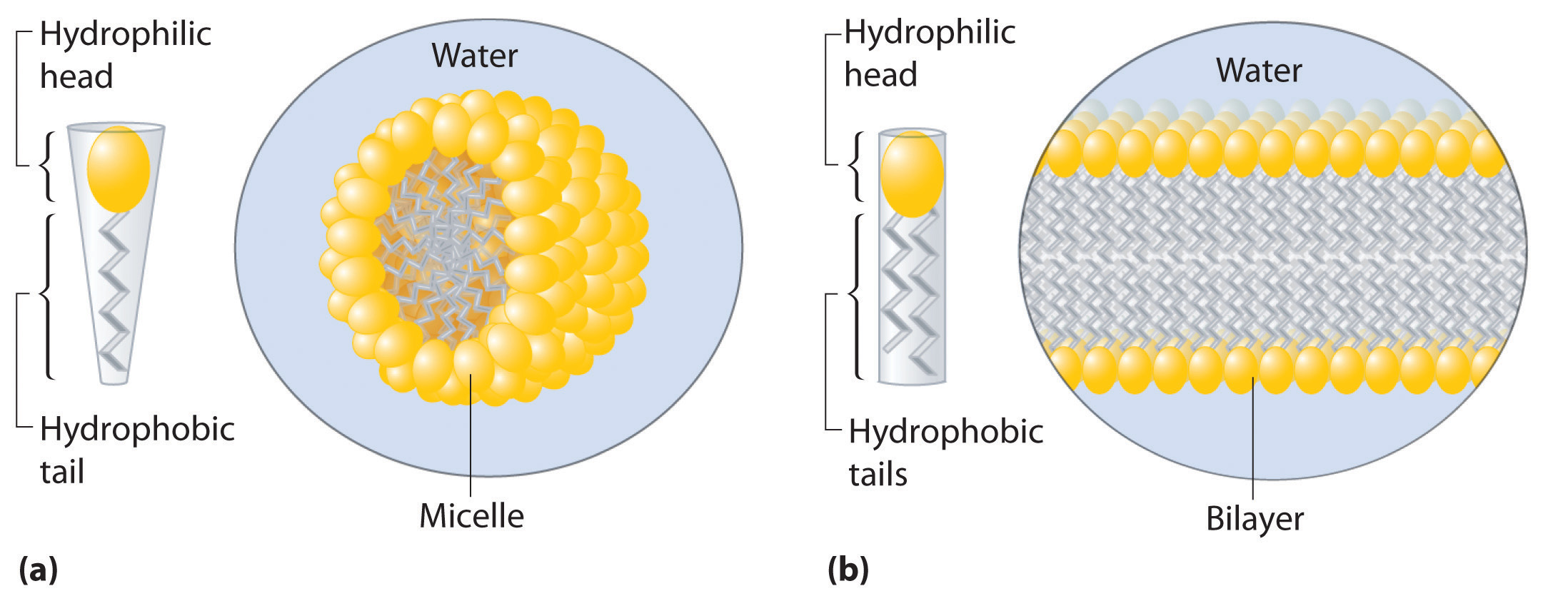
Way beyond VCEAbsolutely don't need to know this, but it has to do with the shape of the molecule.
If you look at a phospholipid, the head of the phospholipid has the same cross-sectional area as the fatty acid tail. In other words, the phospholipid is kind of like cylinder shape. Detergents, on the other hand, are a bit different. The cross-sectional area of the hydrophilic head is greater than the cross-sectional area of the tail. As such, detergents are cone-shaped.
Now that's all well and good, but what does it mean? Well it means that phospholipids like to pack in together in parallel. In other words, they like to line up in a line. Therefore, make a membrane, wherein all the heads can be in water and the tails in lipid, they form a bilayer.
Detergents, on the other hand, like to line up with each other, however, they can't really fit in together side-by-side. So rather than lining up in a straight line, they line up in a line that curves (in 2D, obviously these are actually 3D in real-life). As such, to make sure all the tails are with lipids and the heads in water, they're better to just form tight balls rather than a bilayer membrane.
I appreciate that that's all pretty confusing, so if you're completely lost just ignore what I've said and have a look at the image I've attached. You can see that the shapes of the molecules are different and, as a consequence, they pack in together differently.