I'm kind of stuck with this question..
An oxide of Copper is heated in a stream of hydrogen until only the copper remains according ti the equation:
CuxO(s) + H2(g) --> XCu(s) + H2O(l)
the data for the experiment are given in the table below. Calculate the empirical formula of the oxide of copper.
Item and mass:
Crucible - 27.002g
Crucible plus contents before heating - 27.128g
Crucible plus contents after heating - 27.114g
Alright so if we want to find the mass of CuxO then we subtract the mass of crucible with contents before heating with crucible to find that out (27.128 - 27.002 = 0.126g)
To find the mass of copper then we subtract the other one with the mass of the crucible for the weight of Copper (27.114 - 27.002 = 0.112g).
As a result we have the masses of CuxO and Cu so now we can find the weight of oxygen (0.126-0.112=0.014g)
Now to find the empirical formula then we must find the number of moles for Copper in CuxO and the number of moles of Oxygen in CuxO. In this case we'll be dividing the masses of Copper and Oxygen by their molar mass.
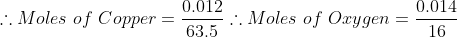
Now the next step in finding the empirical formula is t divide all the amounts by the smallest number of moles. In this case we will be dividing the moles of oxygen against copper to determine our empirical formula (since it's the least).
Keeping our fractions before and dividing, you should get an answer of 256/127 which roughly translates to 2.
Therefore for every one oxygen, there are 2 copper atoms.
So then x=2.
(You can kind of see the answer since the charge of oxygen is -2 so we can assume that the charge of Copper must be equal with it).

